karl fischer volumetric titration procedure solution|karl fischer titration pdf : discount store The determination of the water content according to Karl Fischer is nowadays performed by two different techniques: Volumetric Karl Fischer Titration, where a solution containing iodine is added using a motorized piston burette. Resultado da We would like to show you a description here but the site won’t allow us.
{plog:ftitle_list}
WEBAvatar: The Way of Water. Jake Sully lives with his newfound family formed on the planet of Pandora. Once a familiar threat returns to finish what was previously started, Jake must work with Neytiri and the army of the Na'vi race to protect their planet. Role. Credit.
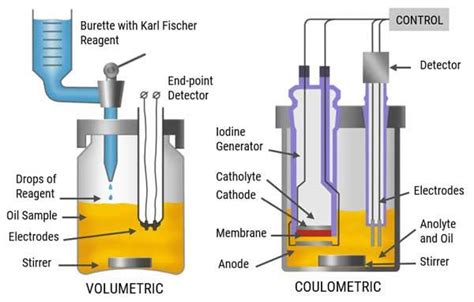
karl fischer volumetric vs coulometric
measures thick dick
The determination of the water content according to Karl Fischer is nowadays performed by two different techniques: Volumetric Karl Fischer Titration, where a solution containing iodine is added using a motorized piston burette.The guide series for Karl Fischer Titration includes the following content: 1 .This Application Bulletin gives an overview of the volumetric water content determination according to Karl Fischer. Amongst others, it describes the handling of electrodes, samples, .In analytical chemistry, Karl Fischer titration is a classic titration method that uses coulometric or volumetric titration to determine trace amounts of water in a sample. It was invented in 1935 by the German chemist Karl Fischer. Today, the titration is done with an automated Karl Fischer titrator.
The Karl Fischer (KF) Titrator is exclusively used for the quantification of water. In a volumetric KF titration, the iodine solution, also known as the “titer”, will react with a sample that contains .
In volumetric Karl Fischer, iodine is added mechanically to a solvent containing the sample by the titrator’s burette during the titration. Water is quantified on the basis of the volume of Karl .The optimum pH range of the sample solution for efficient Karl Fischer titration is between pH 5.5 and 8. When the pH is greater than 8.5, the reaction rate increases due to chemical side reactions. . This is then titrated using the .Whatever your requirements – we have the right Karl Fischer titrator for you Our portfolio of Karl Fischer titrators includes compact all-in-one options for routine moisture determination as well as fully automated Karl Fischer systems for .188005 Aquastar ® - CombiTitrant 5 - One component reagent for volumetric Karl Fischer titration, . Aliquote of 1 mL sample solution. Procedure: The sample solution is prepared by addition of 5 g of sample to 50 mL of pyridine and subsequently filled up with methanol to 100 mL. The Karl-Fischer reagent is placed into the cathode and anode .
The Karl Fischer (KF) Titrator is exclusively used for the quantification of water. In a volumetric KF titration, the iodine solution, also known as the “titer”, will react with a sample that contains water in a methanolic solution until all the water is consumed. Before proceeding with a sample measurement, ensure the Titer concentration has
Volumetric Karl Fischer titration capable of titrating water concentrations from 0.01 – 100%. . The KF solvent is now mixed with the sulfur dioxide and base and the solution is added to the .Karl Fischer titration is used for many substances as a reference method, and is a chemical analysis based on the oxidation of sulfur dioxide by iodine in a methanolic hydroxide solution. In principle, the following chemical reaction takes place: H 2 O + I 2 + SO 2 + CH 3 OH + 3RN -> [RNH]SO 4 CH 3 + 2[RNH]I The titration can be performed .• Titrator with a mode for volumetric Karl Fischer titration • Analytical balance (minimum resolution 0.1 mg) Accessories . reagent solution. 3 After the addition of the standard, place the weighing spoon on the balance again. 4 Enter the added sample weight in the software.
karl fischer titration reagent
samples, and water standards. The described procedures and parameters comply with the ASTM E203. Instruments . Double Pt wire-electrode (indicator electrode for volumetric Karl Fischer titration) Reagents For volumetric KF titration, there is a general distinction between one- and two-component reagents. . different solutions, the titrant .Karl Fischer titration is a titration method that determines the quantity of water present in a given analyte using volumetric or coulometric titration.. Karl Fischer, a German scientist, invented this method for quantitative chemical analysis in 1935.; To perform such titrations, specialist titrators (known as Karl Fischer titrators) are presently available.Karl Fischer titration for water determination is probably the best known and most widely used titration method. The reaction mechanism is well explored and there is a broad offering of suitable reagents and instruments to be used. . With hygroscopic samples fast working procedures are needed and the samples have to be kept protected in .As shown in Formula (1) below, the Karl Fischer method uses Karl Fischer reagent, which reacts quantitatively and selectively with water, to measure moisture content. Karl Fischer reagent consists of iodine, sulfur dioxide, a base and a sol
industrial water-glycols studied. When combined with coulometric Karl Fischer 228.545 titration instead of volumetric Karl Fischer titration, the automated procedure excels at the analysis of low-molecular-weight hydrocarbons such as n-pentane. Lastly, automated sample measurement is a powerful technique to automate a
Difference Between Volumetric and Coulometry Compare the Difference Between Similar Terms. KF titration, volumetric and coulometric. In short, the two methods can be compared as follows: Volumetric Method. Reagent Type. The volumetric method uses a KF reagent containing sulfur dioxide and iodine, which also contains a hydroxyl group and a base.Scope 1.1 This test method is intended as a general guide for the application of the volumetric Karl Fischer (KF) titration for determining free water and water of hydration in most solid or liquid organic and inorganic compounds. . for detailed information concerning toxicity, first aid procedures, and safety precautions for chemicals used .
In volumetric Karl Fischer titration, the endpoint is detected by a change in the electrical conductivity of the solution. In coulometric Karl Fischer titration, the endpoint is reached when the amount of electricity required to generate iodine in the reaction is stoichiometrically equivalent to the amount of water present in the sample.For strongly alkaline amines the buffer capacity of the Karl Fischer solution is not sufficient. A shift of pH into the alkaline range leads to a side reaction of the iodine. . Solvent for volumetric Karl Fischer titration with one component reagents, max. 0,01 % water. . Procedure: The Karl-Fischer reagent is placed into the cathode and .For volumetric Karl Fischer titration, there are mainly two types of reagent. . In this titration, a solution containing iodine is added directly from the burette during titration. Coulometric karl fischer titration. The coulometric Karl .The volumetric Karl Fischer titration is used for samples with a water content greater than 1% in solid and liquid . • Reagents for normal or standard procedure (table 1). These are the most commonly used . and having the necessary reagents in a single solution. Products for volumetric Karl Fischer titration. 5 Table 1: One-component .
This Titration Competence Guide is dedicated to the water content determination by Karl Fischer (KF) titration. Focus has been put on the practical aspects rather than on theoretical knowledge.Titration cell of a standard volumetric Karl Fischer (KF) automatic titrator. The burette injects the KF reagent and this pre-treats the solvent; the sample port allows the addition of the sample with the same procedure. A double platinum electrode measures the controlled-current potentiometry to establish the endpoint.
• A sample extraction procedure is preferred. . the reaction is produced by anodic oxidation in an iodide-containing solution within the titration vessel. • When all the water in the test article has been consumed, an excess of iodine occurs, which is . • For volumetric Karl Fischer titration, linear titra-• Titrator with a mode for volumetric Karl Fischer titration • Analytical balance (minimum resolution 0.1 mg) Accessories . reagent solution. 3 After the addition of the standard, place the weighing spoon on the balance again. 4 Enter the added sample weight in the software. The Determination of Water content in Bio-oils by Volumetric Karl Fischer Titration Analytical Methods . (all reactants are in one solution). The recommended titrant is CombiTitrant 5 which contains base (imidazole), iodine (I . Karl Fischer Instrument. Adjust the procedure accordingly for other instruments. The common base used in Karl Fischer titration is pyridine, primary amines such as imidazole can also be used. . Procedure You can use the Karl Fischer reagents in both Volumetric and Coulometric titrations. In the Volumetric Titration, you use a KF Solution containing dissolved iodine as the titrant until the presence of a trace excess .
Sulphonic acids affect KF solution pH during titration; specific buffers are needed for thiophenes or mercaptans. . CombiMethanol - Solvent for volumetric Karl Fischer titration with one component reagents, max. 0.01 % water. . Procedure: The titration medium is first placed into the titration cell and titrated dry by means of the titrant .Karl Fischer Titration KF Guide 4 An Overview Karl Fischer The Method at a Glance. 2 Editorial . Titration Methods 11 2.1 Volumetric methods 11 2.2 Coulometric methods 13 3. Sample Preparation and Input 15 . Remove aliquots of extraction solution over membrane filter with syringe. Titrant: two component reagent 5 mg H2O/mLIn the original titrimetric solution, known as Karl Fischer Reagent, the sulfur dioxide and iodine are dissolved in pyridine and methanol. The test specimen may be titrated with the Reagent directly, or the analysis may be carried out by a residual titration procedure. The stoichiometry of the reaction is not exact, and the reproducibility of a determination depends upon such .

Página Inicial > Centro de Férias Sesc Bertioga. Localizado em uma área encantadora, no litoral norte de São Paulo, o Centro de Férias Sesc Bertioga é mais do que um hotel. Oferece várias atividades de lazer para que você não precise sair dali para se divertir. Na área do hotel, o destaque é a bela reserva natural.
karl fischer volumetric titration procedure solution|karl fischer titration pdf